| HEAD OF TEAM | : | Prof. Dr. Johnner Sitompul |
| TEAM MEMBERS | : | C.B. Rasrendra, Ana Kemala Putri Jauhari |
| OFFICIAL ADDRESS | : | Laboratorium of Design Method and Process Control |
| : | sitompul@che.itb.ac.id | |
| EXTENDED ABSTRAct | : |
INTRODUCTION
Oil palm carries an extensive readily-to-utilize potential in various industries. As a commonly prevalent solid waste in oil palm industries, Oil Palm Empty Fruit Bunch (OPEFB) can prospectively be re-capitalized as a raw material to produce lactic acid through catalytic chemical reaction. Lactic acid serves to become a vital starting source to produce Poly-D,L-Lactic Acid (PDLLA). To be used as PDLLA, then lactic acid which once must have high purity. Thus, one important stage of the conversion process of OPEFB to PDLLA is the purification stage. The general objective is to produce PDLLA from OPEFB through esterification-hydrolysis purification method. OPEFB chopped with different size ranging from 30 – 60 mesh was introduced into reactor as feed.
METHODOLOGY
Principally, the research methodology can be broken down into four primary stages: (1) pretreatment process of OPEFB with alkaline hydrothermal method; (2) lactic acid production process through catalytic reaction; (3) purification of lactic acid by esterification-hydrolysis method; and (4) production of PDLLA through a direct poly-condensation process. This research puts much emphasis upon the mass balance of OPEFB conversion process into PDLLA.
RESULTS AND DISCUSSION
Based on Table 1, pretreatment using alkaline hydrothermal method can increase cellulose and hemicellulose content and decrease lignin and extractive content. According on Table 1 it can be seen that cellulose and hemicellulose content gradually increased to 46.41%-wt and 24.72%-wt. Meanwhile, the alkaline hydrothermal method was able to decrease the lignin and extractive content successively to 19.72%-wt and 9.15%-wt. During the pretreatment process performed, there is a missing mass OPEFB. In this study, the mass of OPEFB lost during the pretreatment process was 25.48% -wt.
Table 1. Composition of OPEFB untreated and pretreated
| No. | Pretreatment | Cellulose | Hemicellulose | Lignin | Extractive | Source | ||
| (%-m/m) | (%-m/m) | (%-m/m) | (%-m/m) | |||||
| Untreated OPEFB (physical treatment only) | ||||||||
| 1 | Ball-milled + blender (30-60 mesh) | 29,37 | 14,39 | 22,66 | 33,58 | Jauhari, 2017 | ||
| Pretreated OPEFB | ||||||||
| 2 | 0,5 M NaOH + hydrothermal | 46,41 | 24,72 | 19,72 | 9,15 | Jauhari, 2017 | ||
| 3 | Boilling (100oC) + 0,5 M NaOH + hydrothermal | 51,20 | 23,47 | 22,13 | 3,20 | Turnip, 2017a | ||
| 4 | Boilling (100oC) + 0,5 M NaOH + hydrothermal + bleaching | 71,33 | 6,67 | 5,33 | 16,67 | Turnip, 2017b | ||
| 5 | Steam explosion | 63,57 | 17,3 | 9,69 | 9,44 | Aini, 2017 | ||
Based on Figure 1, it can be compared that the byproducts produced by using Ba(OH)2 catalysts are more diverse than using PbCl2. Meanwhile, according to Figure IV.9, the composition of byproducts of organic acid produced by using Ba(OH)2 catalyst was more than using PbCl2 catalyst. Organic acid byproducts are made up of formic acid, acetic acid, and levulinic acid. The use of a Ba(OH)2 catalyst yields three organic acids with a greater composition than using a PbCl2 catalyst. According on Figure 1, the PbCl2 catalyst does not produce formic acid as a by-product of the catalytic reaction of OPEFB.
Figure 1. Comparison of organic acid composition with Ba(OH)2 and PbCl2 catalysts
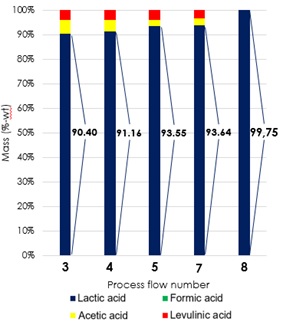
Figure 2 presents a change in the organic acid composition contained in the separation product of each step. Based on Figure 2, the lactic acid content at each stage of the separation process varies and continues to increase in the subsequent separation step. At the end of the separation process, when compared with other organic acid content, the final product obtained has a lactic acid composition of 100%-wt. Each stage of the process gives a different effect on each organic acid involved. At the end of the separation process, lactic acid can be recovered with a recovery percentage percentage of 72.67%-wt. Through this method of separation, acetic acid and levulinic acid can be completely separated from the product.
Figure 2. Changes of organic acid composition
Table 2. Comparison of the PLA molecular weight in several studies
| No. | Lactic acid composition | Molecular weight of PLA (gram/mole) | Condition and catalyst | Source |
| 1 | 60% LA fermentation and 40% LA catalytic | 76,489 | 180oC (50 torr), 30 h, ZnO as catalyst | Jauhari, 2017 |
| 2 | 100% LA catalytic | 150,616 | 180oC (10 torr), 30 h, ZnO as catalyst | Prasetyo and Hermawan, 2013 |
| 3 | 100% LA catalytic | 98,657 | 180oC (50 torr), 30 h, ZnO as catalyst | Abednego and Ivory, 2017 |
| 4 | 100% LA catalytic | 36,000 | 180oC (50 torr), 30 h, ZnO as catalyst | Ren, 2010 |
Based on Table 2 above, the molecular weight of PDLLA synthesized from a fermentation and catalytic lactic acid mixture, having a molecular weight of 76489 grams / mol. The molecular weight of this produced PDLLA does not differ greatly from the molecular weight of PDLLA synthesized from 100% fermentation lactic acid. The synthesized PDLLA molecular weight of 100% commercial lactic acid has a molecular weight range of 79394 – 98657 grams/mole. The weight of the PDLLA molecule obtained in this study is still beyond the PDLLA molecular weight range of 100% commercial lactic acid synthesis. However, the weight of this obtained PDLLA molecule is not much different from the lower range of the molecular weight of PDLLA synthesis of 100% of the commercial lactic acid. Thus, the quality of PDLLA obtained can be said to be quite good as PDLLA which is synthesized by using direct polycondensation method.
According to Table 3, the yield of synthesized PDLLA from fermentation and catalytic lactic acid mixtures has a gain of 9.71%-wt. This value is much smaller than that of other PDLLA. The largest PDLLA obtained is 50.61%-wt obtained by using 100% fermentation lactic acid.
Table 3. Comparison of PLA yield in several studies
| No. | Lactic acid composition | Yield of PLA (gram/mole) | Condition and catalyst | Source |
| 1 | 60% LA fermentation and 40% LA catalytic | 9.71 | 180oC (50 torr), 30 h, ZnO as catalyst | Jauhari, 2017 |
| 2 | 100% LA catalytic | 35 | 180oC (10 torr), 30 h, ZnO as catalyst | Prasetyo and Hermawan, 2013 |
| 3 | 100% LA catalytic | 23.68 | 180oC (50 torr), 30 h, ZnO as catalyst | Abednego and Ivory, 2017 |
| 4 | 100% LA catalytic | 35 | 180oC (50 torr), 30 h, ZnO as catalyst | Ren, 2010 |
CONCLUSIONS
The experiments show that alkaline hydrothermal can improve the cellulose content up to 46.41%-m/m. The highest lactate acid yield in the catalytic reaction was obtained using 0.026 M concentration of PbCl2 catalyst, with a yield attaining 89.45%-m/m. At the end of the purification process, lactic acid product with concentration of 73.36%-m/m was produced, leaving Pb2+ content at a level of 3.29 ppm. Ultimately, the yield of lactic acid at end of purification process was 65.01%-m/m, whereas the yield of PDLLA at the end of the direct poly-condensation process is 7.96%-m/m. The obtained PDLLA has a molecular weight of 76,489 grams/mol.