| HEAD OF TEAM | : | Prof. Ir. Yazid Bindar, M.Sc., Ph.D |
| TEAM MEMBERS | : | Pasymi ST., MT. |
| OFFICIAL ADDRESS | : | Energy and Processing System on Chemical Enginnering Research Group, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung, Indonesia |
| : | yazid@che.itb.ac.id | |
| EXTENDED ABSTRAct | : |
Renewable fuel production from vegetable oil and fat or its fatty acids by direct decarboxylation has been widely reported. An innovative approach to produce drop-in fuel via thermal catalytic decarboxylation of basic soap derived from palm stearin reported in this research. The catalytic effect of the calcium and magnesium metals in the basic soap and its decarboxylation on drop-in fuel yield and product distribution was studied. The catalytic effect was tested in the temperature range up to 370oC and atmospheric pressure for 5 hours in a batch reactor. It has been proved that the calcium basic soap decarboxylation, effectively produce the drop-in fuel in carbon ranges C8 – C20, in which more than 78% selectivity toward alkane. Whereas, only 70% selectivity toward alkane has been resulted from the magnesium basic soap decarboxylation.
Pendahuluan
Nowadays, a considerable of attention has been devoted to producing renewable diesel (drop-in fuel) via deoxygenation of vegetable oils and fats or fatty acids. One promising deoxygenation method for the production of drop-in fuels is basic soap decarboxylation. Decarboxylation of basic soaps (e.g., magnesium soap) into drop-in fuels, illustrated by the equation of reaction 1.
(R-COO)Mg(OH) à RH + MgCO3 (1)
The presence of the 1-alkene and the ketone component in the fuel product, related to the character of the magnesium basic soaps decomposition. Both 1-alkene or ketone, are not expected in all drop-in fuel, because it causes the fuel instability. Therefore, the metal for preparing of the basic soap, must be carefully selected, which have high catalytic and selectivity ability to form n-alkanes during the basic soap decarboxylation. The purpose of this study is to prove that the decarboxylation of reactants from calcium basic soaps derived from palm stearin, can produce high green diesel content of n-alkanes and free ketones.
Methods
The Mg and Ca basic soap were prepared by metathesis process. Decarboxylation reaction were performed in a glass reactor (100 ml) operating in a semi batch mode and at atmospheric pressure. Decarboxylation proceeded at temperatures up to 370oC for 5 hours. The liquid reaction products were analyzed by gas chromatograph (GC).
Results and Discussion
Product yields from decarboxylation and Qualitative analysis of liquid hydrocarbon
Table 1. Material balance for the reaction product.
| Material | Yield (wt % of feed) | |
| Mg base | Ca base | |
| liquid biohydrocarbons | 67.21 | 63.33 |
| water | 1.14 | 1.42 |
| solid residues | 28.23 | 32.93 |
| gas | 3.42 | 2.32 |
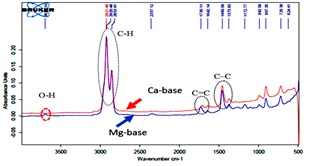
Fig. 1. FTIR of liquid biohydrocarbon from basic soap: red line, Ca base, and blue line, Mg base.
Table 1 shows that the liquid biohydrocarbon resulted of Mg-basic soap is 67.21%-wt, greater than 63.33%-wt of Ca basic soap. The Ca basic soap decarboxylation result the most solid residues product (i.e., 32.93%-wt) than its counterpart Mg (28.23% -wt). The gas products by Ca-basic soap less than Mg-soap. This implies that MG-basic soap is faster decomposed to light products than ca. According to GC-TCD chromatogram, the gases generated from both Mg and Ca basic soaps contain H2, CO2 and CH4.
Figure 1 shows that the liquid product was resulted from both the Mg and Ca basic soap decarboxylation contains the C-C, C = C and C-H functional groups in the range 1375-1460, 642-1730, and 2853-2921 cm-1, respectively. Whereas, the hydroxide (O-H) group at the wave number 3618 cm-1, only detected in the liquid product of the Mg-basic soap. The results mentioned above show that oxygenated compounds still exist in the liquid biohydrocarbon from Mg basic soap than Ca-basic soap. The GC-MS analysis results, also confirmed that about 2.6% – mole of ketone compounds are present in the liquid biohydrocarbon from Mg basic soap. Whereas, it is not detected in the liquid biohydocarbon from Ca basic soap.
Distribution of liquid products profile and Quantitative analysis: alkane and olefin
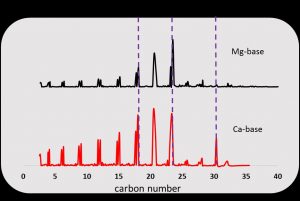
Figure 2 shows that the decarboxylation of Mg and Ca basic soaps produce liquid biohydrocarbons in the range of C8-C20 carbon number. According to GC-FID chromatogram the n-pentadecane and n-tetradecane was the main product in the decarboxylation of both Mg and Ca basic soap. However, few of the 1-pentadecene also was formed in the decarboxylation of Mg basic soap. In case of n-octadecane, significantly were only formed in the decarboxylation of Ca basic soap. It means that Ca can catalyze the polymerization reaction of short-chain into long-chain liquid biohydrocarbon molecules. Figure 2 also shows that the liquid biohydrocarbon product contains alkane (consisting of n-alkanes and iso-alkanes) and alkene compounds. In case of long chain alkanes (e.g., C14, C15 and C18) are higher produced in the decarboxylation of Ca basic soap than Mg basic soap. Whereas, in case of the C8-C13 product, always produced n-alkane and 1-alkenes compounds in the relatively equal peak height in both basic soap.
Fig. 2. GC-FID chromatograms patterns for liquid bio-hydrocarbon fractions.
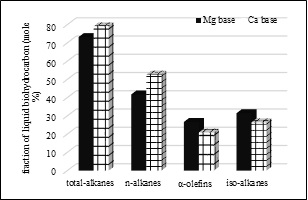
Fig. 3. percentages of C8-C20 total alkanes, n-alkanes, iso-alkanes and α-olefins formed.
Figure 3 shows that, about 78%-mole of liquid biohydrocarbons produced from the decarboxylation of the Ca basic soaps are alkane compounds (i.e., n-paraffin + iso-paraffin), as would be expected in green diesel. The rest (approxymately 20%-mole) are various α-olefin molecules. Whereas, only about 70%-mole of alkanes resulted of Mg basic soap. Therefore, the resulting α-olefin of Mg basic soap is high, i.e., about 27%-mole or 7%-mole more than the same product of Ca basic soap.
Figure 4 shows that the molar ratio of n-alkanes to 1-alkene is found to be higher for C14, C15, C18 and C19 in the liquid product of Ca basic soap. This shows that the decarboxylation of Ca basic soap produces the n-alkane more than 1-alkene fraction for C14, C15, C18 and C19 molecules. In other words, Ca has high selectivity toward the formation of long chain n-alkane products, as which are expected to green diesel fuel. The more balanced the amount of the n-alkanes and 1-alkenes in the liquid product, its molar ratio close to one, as indicated by the C8-C11 molecules.
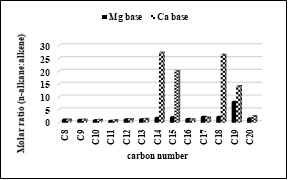 Fig. 4. The molar ratio of n-alkanes to 1-alkenes.
Fig. 4. The molar ratio of n-alkanes to 1-alkenes.
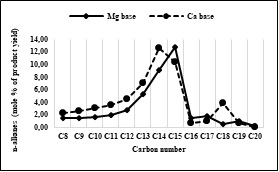 Fig. 5. Molar yield of C8-C20 n-alkanes at 370oC for 5 hours.
Fig. 5. Molar yield of C8-C20 n-alkanes at 370oC for 5 hours.
Figure 5 shows that about 10%-mole and 13%-mole of 53%-mol total of n-alkane fraction (in the carbon number range C8-C20) resulting from decarboxylation of a Ca basic soap are n-pentadecane (n-C15) and n-tetradecane (n-C14), respectively. Whereas, only about 13%-mole and 9%-mole of 42%-mol total n-alkanes, generated from Mg basic soap are n-pentadecane and n-tetradecane, respectively. From the total yield of n-alkane products were resulted from Ca basic soaps, about 29%-mole is a long chain alkane (C14-C18) suitable for green diesel, while only about 26%-mole of Mg basic soap. The n-octadecane product is detected in significant amounts (about 4%-mole), only in the liquid product of decarboxylation of the Ca basic soap. Apparently, the Ca may act as a polymerization catalyst of short chain to long chain biohydrocarbons.
Conclusions
Mg and Ca basic soaps, can be converted to liquid biohydrocarbon or drop-in fuels, especially green diesel via decarboxylation method. Green diesel generated from Ca basic soaps, contains 78%-mole total alkanes, higher than Mg basic soap (only about 70%-mole). No ketone compound is detected in green diesel produced by calcium basic soap. The product distribution, in particular the concentration of the saturated C14 and C15 biohydrocarbons, is significantly higher in the green diesel produced by Ca basic soaps. This proves that, the use of Ca in basic soaps, significantly increases the selectivity to long chain n-alkane products (especially C14, C15 and C18), which are expected by green diesel fuel.