| HEAD OF TEAM | : | Prof. Dr. Herri Susanto |
| TEAM MEMBERS | : | Joko Waluyo, ST., MT |
| OFFICIAL ADDRESS | : | Lab TSU – Chemical Engineering Dept – Labtek X ITB |
| : | herri@che.itb.ac.id | |
| EXTENDED ABSTRAct | : |
Tar accumulation can result in blockage, corrosion, and poisoning of catalyst. One of the methods applicable for tar removal is by using a catalyst that enhances steam reforming reaction. In this study, natural zeolites were modified for the use as tar steam reforming catalyst. Zeolite modification can be performed by conducting ion exchange, acid leaching, alkaline leaching or hydrothermal treatment. In this study, the natural zeolite is modified by ion exchange and acid leaching. Acid leaching are performed with the intention of removing impurities and also possibly increasing the surface area through dealumination. The dealumination process causes increased thermal resistance and decreases the acidity of the zeolite. The addition of nickel is expected to increase the activity of the zeolite as a steam reforming catalyst. The purpose of this study is to determine the effect of acid leaching duration and the presence of nickel on the characteristics of zeolite and its activity.
This study also covers characterizing the modified natural zeolite by ion exchange and acid leaching, and measuring its activity as toluene steam reforming catalyst. The XRD pattern showed that natural zeolite contains three crystalline phases, i.e. mordenite, clinoptilolite, and heulandite (Figure1). The loss of some zeolite impurities especially Na and K which have been replaced with H+ cations, such as the results of elemental composition analysis presented in Table 1.
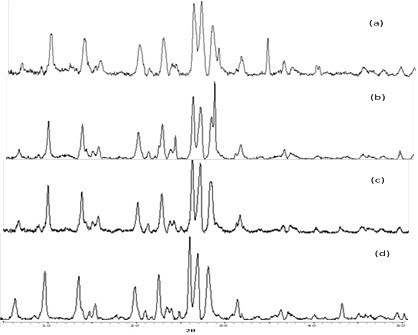
Figure 3. XRD pattern of (a) parent zeolite, (b) after ion exchange ZH, (c) after acid leaching 3 hr, ZM3 and (d) after acid leaching 6 hr, ZM6
Table 1. Elemental composition as determine by EDX analysis
| No | Sample | Si | Al | Na | K | Mg | Ca | Fe | Ti | O | C | Ni |
| 1 | ZF | 29.75 | 7.09 | 0.72 | 0.65 | 0.87 | 3.06 | 2.57 | 0.27 | 55.02 | 0 | 0 |
| 2 | ZH | 32.47 | 7.85 | 0.42 | 0.17 | 0.53 | 2.15 | 2.21 | 0.34 | 51.31 | 2.55 | 0 |
| 3 | ZM3 | 31.48 | 7.23 | 0.22 | 0 | 0.53 | 1.72 | 1.66 | 0.27 | 51.21 | 5.68 | 0 |
| 4 | ZM6 | 31.68 | 6.71 | 0.22 | 0 | 0.58 | 1.75 | 2.01 | 0.34 | 51.19 | 5.52 | 0 |
| 5 | ZM6Ni5 | 31.01 | 6.28 | 0.19 | 0 | 0.36 | 1.67 | 0.72 | 0 | 50.2 | 4.24 | 5.34 |
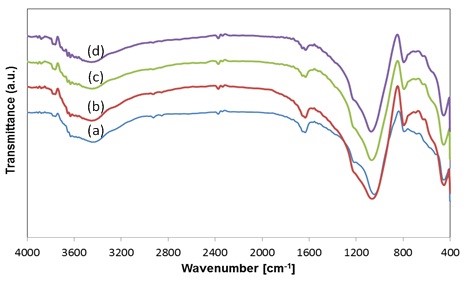 Figure 2. FTIR spectrum of (a) parent zeolite, (b) ZMH, (c) ZM3 and (d) ZM6
Figure 2. FTIR spectrum of (a) parent zeolite, (b) ZMH, (c) ZM3 and (d) ZM6
The main structure of the mordenite phase was also detected by using FTIR analysis on T-O stretching band with the range of 1045-1075 cm-1 (Figure 2). The zeolite modification by ion exchange and acid leaching resulted in decreasing content of impurities, such as Na and K, and the decrease in crystallinity from 68.8% to 61.8%. The surface area increased from 16 m2/g to 31.4 m2/g and 161.8 m2/g for ZMH (after ion exchange), ZM3 (after acid leaching for 3 hour), and ZM6 (after acid leaching for 6 hour) respectively. Modifying the natural zeolite with mordenite as the main phase, through ion exchange and acid leaching can increase the conversion of toluene cracking by reaction of steam reforming. Conversion of toluene with the modified natural zeolite can reach up to 54%, this is due to modification by acid leaching can increase the surface area of zeolite mordenite, and the toluene will easily react with steam. Acid leaching time affects the surface area of the zeolite, the longer the leaching makes the surface area become larger as presented in Table 2.
Table 2. Psychochemical properties of natural zeolite after modification
| No | Zeolite | Si/Al
ratio* |
ABET (m2/g) | Vt (cm3/g) | Crystallinity |
| 1 | ZMH | 5.09 | 16.0 | 0.094 | 66.3% |
| 2 | ZM3 | 5.02 | 31.4 | 0.086 | 63.7% |
| 3 | ZM6 | 5.61 | 161.8 | 0.159 | 61.8% |
| 4 | ZM6Ni5 (5%Ni) | 5.11 | 23.56 | 0.079 |
*Analyzed by XRF
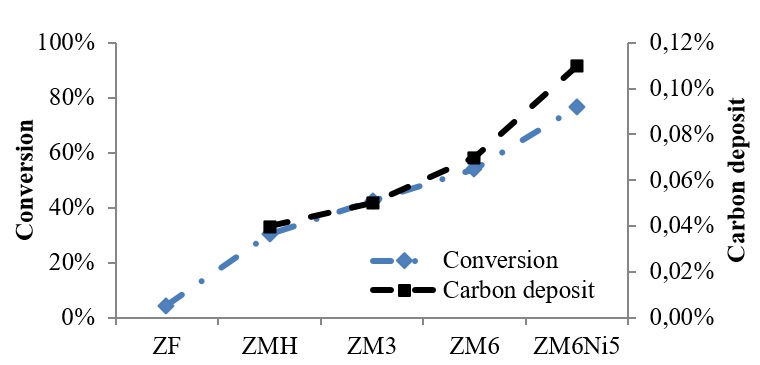
Figure 3. Conversion of toluene and carbon deposits on various zeolites modification
The natural mordenite can be utilized as a catalyst support. By performing nickel impregnation as the active phase on mordenite can increase toluene conversion up to 95% (Figure 3). The presence of nickel in zeolites can decrease the acidity of the zeolite catalyst, thereby increasing the toluene cracking reaction. Advantages of Ni-zeolite catalyst is without the necessary reduction of NiO into Ni. The zeolite has given activity to cracking the tar into H2, which can then be used for the impregnated NiO reduction in the catalyst.
